News / Update
Checklist for First In Human (FIH) Research Protocols
Checklist for First In Human (FIH) Research Protocols Version 3 (Updated on 11th October 2024)
Attachments
Directive For Receiving Application Of CTIL/CTX Involving First In Human Clinical Trial
Arahan Pengarah Kanan Perkhidmatan Farmasi Bilangan 4 Tahun 2019 : Direktif Untuk Menerima Permohonan Lesen Import Percubaan Klinikal (CTIL) & Kebenaran Untuk Mengilang Produk Tidak Berdaftar Untuk Tujuan Percubaan Klinikal (CTX) Yang Melibatkan First In Human
Arahan di Bawah Peraturan 29 , Peraturan-Peraturan Kawalan Dadah & Kosmetik 1984, Akta Jualan Dadah 1952
Arahan di Bawah Peraturan 29 , Peraturan-Peraturan Kawalan Dadah & Kosmetik 1984, Akta Jualan Dadah 1952
Attachments
Health Research Priorities For 12th Malaysia Plan (12MP-HRP) 2021 – 2025
Health research priorities in Malaysia should reflect the unique characteristics, needs and goals of the health sector in Malaysia. The primary goal of Malaysian health sector is to improve the health and quality of life of the people of Malaysia. This in turn, would enhance national productivity and competitiveness, with positive impacts on economic development. The purpose of identifying health research priorities is to facilitate research that provides relevant and targeted information to support the primary goal of the health sector. The resultant research outputs should improve the health status and the delivery of health care in Malaysia. From a social perspective, health research primarily aims to bring social benefits via a variety of means, including, for instance, evaluation of health-related epidemiological data in the Malaysian-setting, and industry-driven development of innovative technology with commercial value. Only some research in health technology might have a direct commercial value, but their development should be anchored primarily on the potential health benefits they will bring to Malaysians. In this document, the selection of priority areas for health research was guided by information/evidence requirements related to two fundamental principles. First, the core principles in the 12th Malaysia Plan (12MP), and second is the continuing need to promote wellness and to reduce the burden of current and emerging diseases among Malaysians.
Attachments
NIH Guidelines For Conducting Research In Ministry of Health Institutions & Facilities 3rd Edition 2021

Latest Updates - 3rd Edition 2021 . This updated guideline is compiled to help investigators from MOH as well as others who are involved in the conduct of any research involving the MOH institution, facilities, subjects, samples or data.
Attachments
Director General of Health Circular Bil 4/2022 on NIH Guidelines For Conducting Research in Ministry Of Health (MOH) Institutions And Facilities (3rd Edition/2021) 31/01/2022
The latest circular on the conduct of research in Ministry of Health (MOH) Facilities and Institution. This circular together with the new "NIH Guidelines For Conducting Research in Ministry Of Health (MOH) Institutions And Facilities 3rd edition,2021" is effective from the date this circular is issued. The previous relevant circular is thereby automatically void.
Attachments
Guideline For Herbal Medicine Research - 1st Edition 2023

The Guideline for Herbal Medicine Research by the National Commitee for Research and Development of Herbal Medicine (NRDHM) 1st Edition 2023 . This document provides structured guidance to be utilised by researchers, academicians, clinicians, T&CM practitioners, and relevant stakeholders of the herbal industry. This guideline outlines a concise yet informative description of the prerequisites and processes involved in planning and conducting herbal medicine-related research in the Malaysian.
Attachments
Malaysian Decentralised Clinical Trials (DCT) Guidance Document

Malaysia Decentralised Clinical Trials (DCT) Guidance Document is a document endorsed by the National; Committee for Clinical Research (NCCR) . This document provides a guidance on the implementation of decentralized elements in clinical trials with investigational medicinal products, regardless of any health crisis, and with the intention to facilitate use of DCT elements in clinical trials in Malaysia
Attachments
JPP-NIH Panels Meeting Tentative Dates Planner 2024
Attachments
Document Manual Updates for NMRR Submission
Documents on the manual for NMRR Submission. Documents include:
1) Data Elements and Parameters For NMRR Submission (V2.1)
2) Manual for Initial Research Registration Submission and Other Submission Purposes (V2)
3) Manual for Revision Submission and Deletion of Submission (V1)
Click on the document name to download the document for your reference.
1) Data Elements and Parameters For NMRR Submission (V2.1)
2) Manual for Initial Research Registration Submission and Other Submission Purposes (V2)
3) Manual for Revision Submission and Deletion of Submission (V1)
Click on the document name to download the document for your reference.
Attachments
Tatacara Permohonan Geran Penyelidikan KKM
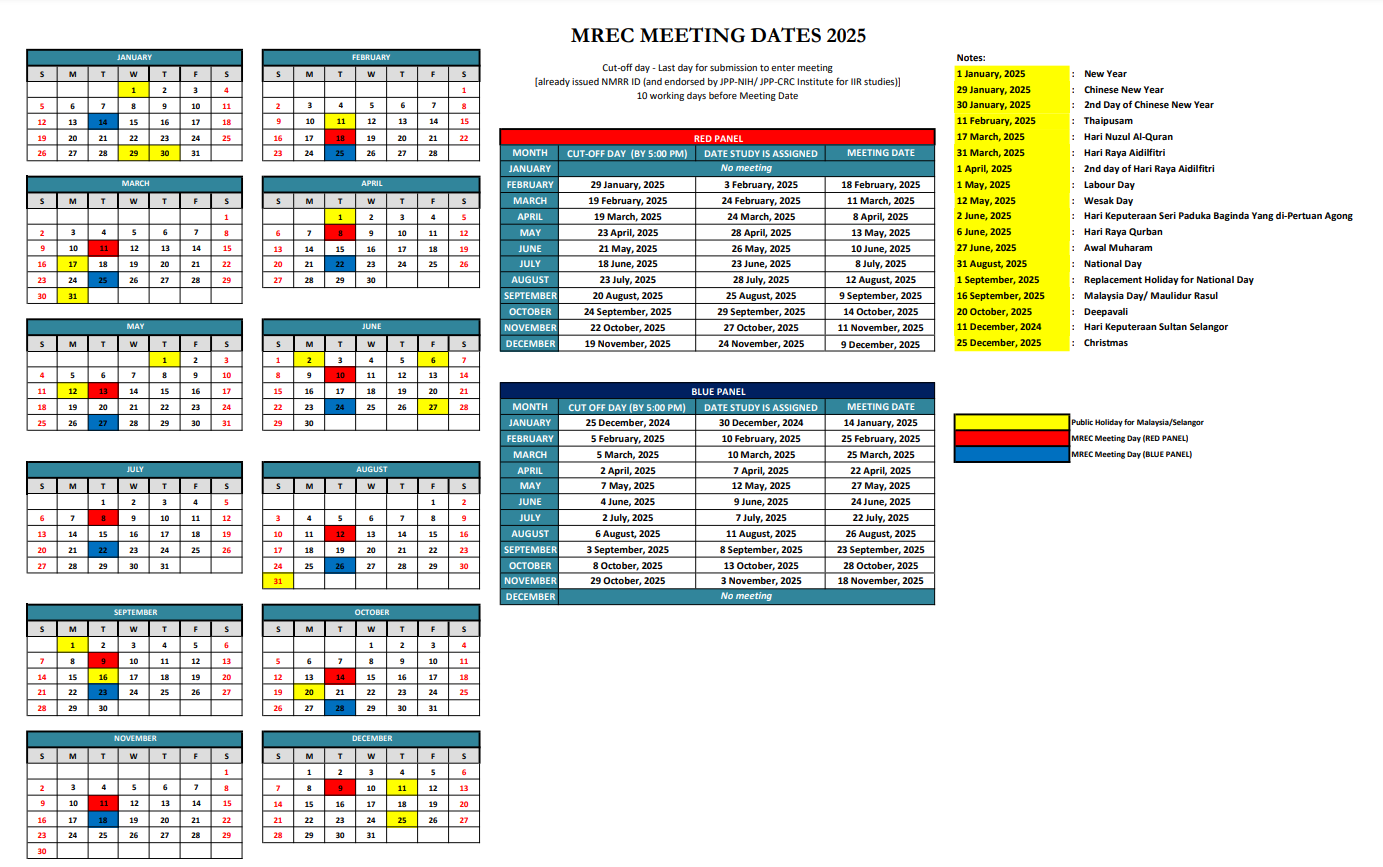
MREC Full Board Panels Meeting Tentative Dates Calendar 2025
Attachments
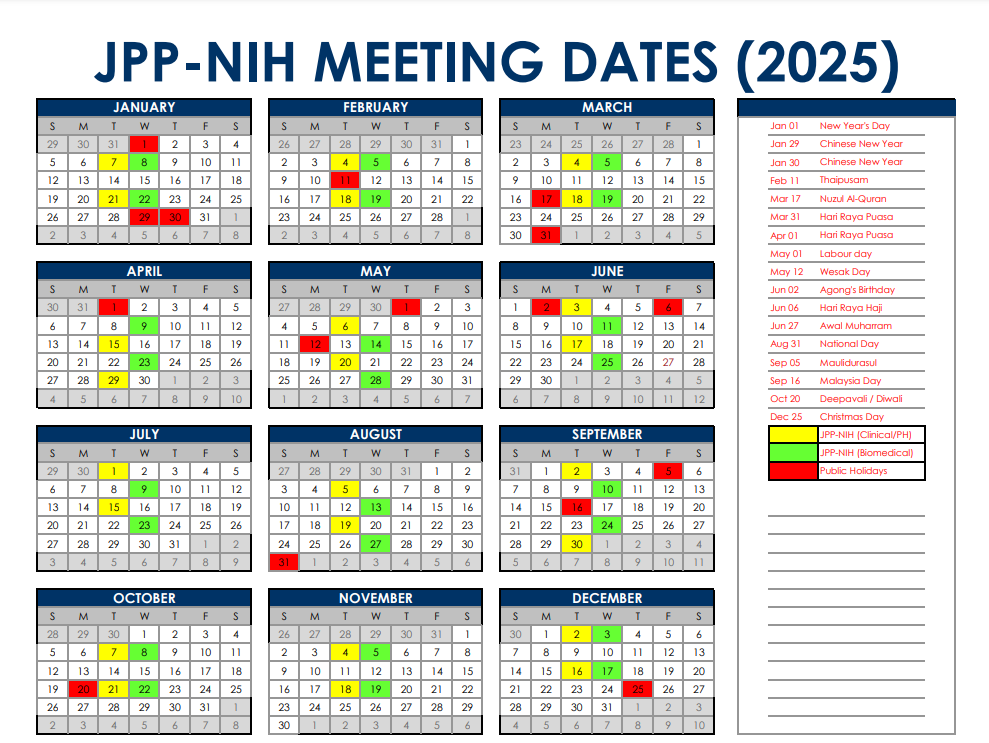
JPP-NIH Meeting Tentative Dates Calendar 2025
Attachments
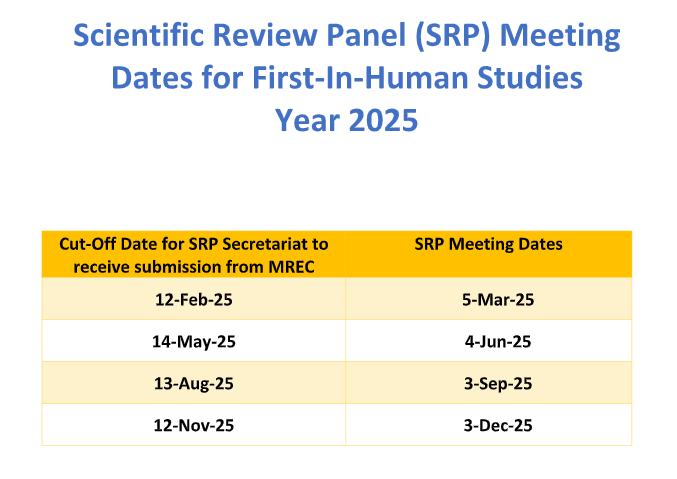
First-in-Human SRP Meeting Dates Year 2025